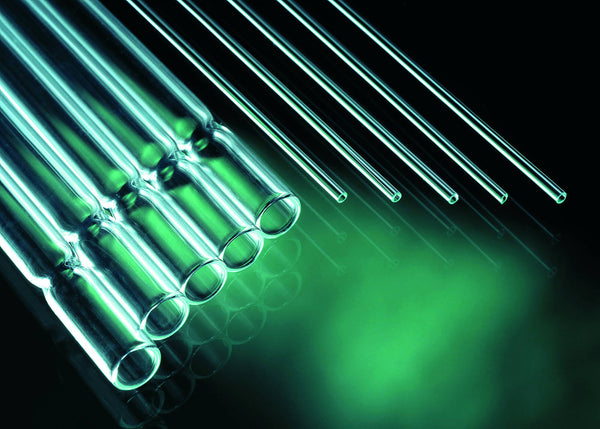
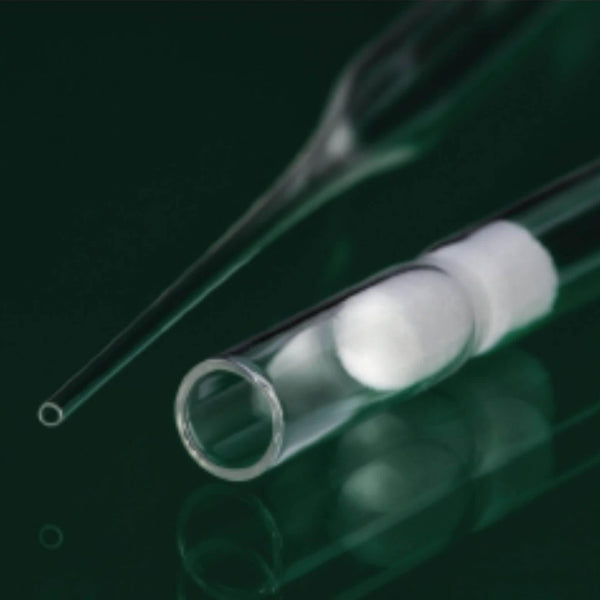
IVF Pasteur pipettes for gamete and media handling
These premium glass Pasteur pipettes are prepared specifically for In Vitro Fertilization procedures. The Premium Pipets are now available in 2 types of Glass.
There is no visible difference in the two glass pipets. The only difference is the color of the outer box. Blue for borosilicate and green for soda glass.
Borosilicate Glass has experienced a world wide shortage of raw materials and Covid Vaccine products have put critical strains on Borosilicate Glass supplies.
- Consistent quality
- Sterile
- Independently laboratory tested
- Mouse Embryo Assay (MEA)
- Human Sperm Motility Assay (HSMA)
- Limulus Amebocyte Lysate (LAL)
- Comprehensive range of products
- Tamper evident Seal
- 100% final inspection
- Superior primary & secondary packaging
Features:
- Thoroughly washed high quality Borosilicate glass
- Validated CE marked product “Intended use” for IVF
- MEA, LAL and HSMA (Human Sperm Motility Assay)
batch release tested - Unique fire polished tips
- Man-made plugs no cotton fibre drop.
- Superior packaging / wider seals on packets / Product doesn’t rattle or move in the packet / Strong boxes and packets for transportation worldwide.
- 100 pipettes per box / 5 per packet
 |
 |
 |
Specifications:
| Mfr. No. PPB150-100 | 5.75” (150mm) Short Borosilicate Pasteur. MEA, LAL and HSMA Tested CE Marked for IVF use. Box of 100 (20 x bag of 5). |
| Mfr. No. PPB230-100 | 9” (230mm) Long Borosilicate Pasteur. MEA, LAL and HSMA Tested CE Marked for IVF use. Box of 100 (20 x bag of 5). |
| Mfr. No. PPB150-100PL | 5.75” (150mm) Short Borosilicate Plugged Pasteur. MEA, LAL and HSMA Tested CE Marked for IVF use. Box of 100 (20 x bag of 5). |
| Mfr. No. PPB230-100PL | 9” (230mm) Long Borosilicate Plugged Pasteur. MEA, LAL and HSMA Tested CE Marked for IVF use. Box of 100 (20 x bag of 5). |
| Mfr. No. PPS150-100 | 5.75” (150mm) Short Soda Glass Pasteur. MEA, LAL and HSMA Tested CE Marked for IVF use. Box of 100 (20 x bag of 5). |
| Mfr. No. PPS230-100 | 9” (230mm) Long Soda Glass Pasteur. MEA, LAL and HSMA Tested CE Marked for IVF use. Box of 100 (20 x bag of 5). |
| Mfr. No. PPS150-100PL | 5.75” (150mm) Short Soda Glass Plugged Pasteur. MEA, LAL and HSMA Tested CE Marked for IVF use. Box of 100 (20 x bag of 5). |
| Mfr. No. PPS230-100PL | 9” (230mm) Long Soda Glass Plugged Pasteur. MEA, LAL and HSMA Tested CE Marked for IVF use. Box of 100 (20 x bag of 5). |
Pasteur pipettes for gamete and media handling
Hunter Scientific conforms to Medical Device Regulations, which enables us to apply the CE mark as a Class IIa Medical Device.
All our Pasteur pipettes undergo a rigorous washing regime, dried and then sterilised.
A special non-cotton material is used for the plugged versions, which avoids cotton or fibre contamination.
All Pasteur pipettes are supplied with uniquely flamed fire polished tips.
The pipettes are packed in pouches of 5.
A sturdy outer shipping box ensures safe transportation and storage. Identifiable by a blue box for Borosilicate glass and a green box for Soda glass.
Under Medical Device Directive, sterile Pasteur pipettes are regarded as Class IIa Medical Devices and are CE marked accordingly.
All products are Toxicity Tested. As a holder of ISO 13485:2016 QMS, we are regularly audited both internally and externally.
About Hunter Scientific
Hunter Scientific is a specialist supplier to the IVF market, and a major manufacturer of Pasteur pipettes. Our products have been developed in collaboration with leading practitioners in the UK
Quality Systems
Hunter Scientific maintains the highest standards for all manufactured and distributed products.
Hunter Scientific is registered to
ISO 13485:2016. All Hunter Scientific manufactured Pasteur pipettes are produced as Class IIa Medical Devices.
They are validated for use under Medical Device Directive 93/42/EEC.
Hunter Scientific manufacture CE marked products and have a positive, pro-active approach to working with suppliers to continuously improve the quality of products in both performance and certification.