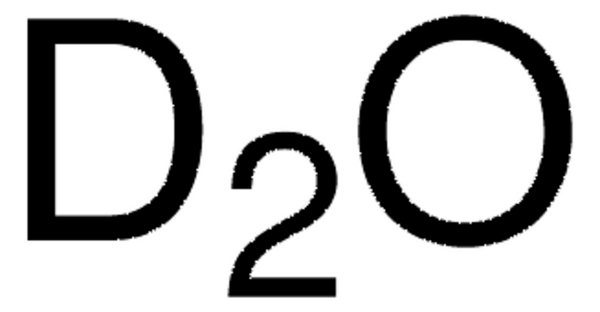Deuterium oxide
General description
Deuterium oxide, a deuterated solvent,[1] is a standard prurity solvent for NMR (Nuclear Magnetic Resonance) analyses. Various thermodynamic properties (such as intermolecular vibrational frequencies, energy of the hydrogen bond, free energy, enthalpy and entropy) of liquid deuterium oxide have been evaluated.[2] Ionization constant for D2O (in the range of 5-50°C), pK values (at 25°C) and enthalpy, entropy, heat capacity change (for the dissociation of D2O) have been reported.[3]
Application
Deuterium oxide (Heavy water, Water-d2, D2O) has been used as solvent for the dissolution of internal standard and sample during quantification experiments by NMR.[1] It has been used for the dissolution of tris(2,2′-bipyridyl)dichlororuthenium(II) hexahydrate Ru(bpy)3.4
Deuterium oxide may be used:
- To prepare trifluoroacetic acid-d by reacting with trifluoroacetic anhydride.[4]
- As a deuteration agent for primary and secondary alcohols at the β-carbon position via H/D exchange reaction in the presence of ruthenium catalyst.[5]
- Along with hexamethyldisilane as a deuterium transfer reagent for alkynes to form (E)-1,2-dideuterioalkenes in the presence of a palladium complex.[6]
Properties
| Isotopic Purity | 99.9 atom % D |
| Quality Level | 200 |
| Form | Liquid |
| Technique(s) |
NMR: Suitable bio NMR: Suitable |
| bp | 101.4 °C (lit.) |
| mp | 3.8 °C (lit.) |
| Storage Temp. | Room temp. |
| SMILES String | [2H]O[2H] |
| InChl | 1S/H2O/h1H2/i/hD2 |
| InChl Key | XLYOFNOQVPJJNP-ZSJDYOACSA-N |
Synonym(s):
Heavy water, Water-d2
Empirical Formula (Hill Notation):
D2O